An in vitro study comparing a two-part activated chlorine dioxide oral rinse to chlorhexidine
Abstract
Background: Chlorhexidine is considered the “gold standard” for antiplaque agents. However, there are side effects associated with long-term use of chlorhexidine. This study compared a chlorine dioxide-based mouth rinse (Oracare) with chlorhexidine for antimicrobial activity and an ability to remove volatile sulfur compounds (VSCs) generated by the periodontal pathogen Porphyromonas gingivalis.
Methods: A standard MIC (minimum inhibitory concentration) test was used to compare the Oracare rinse with chlorhexidine for inhibition of common dental pathogens. The effectiveness of each rinse for removing VSCs generated in vitro by the periodontal pathogen Porphyromonas gingivalis was measured with a halimeter. A rinse with water served as a negative control.
Results: The MICs for Oracare were higher than those for chlorhexidine when compared directly against a panel of 11 microbial species. However, when normalized to parts per million of the active ingredients, the Oracare rivaled the activity of chlorhexidine and exceeded it in activity toward the periodontal pathogen Aggregatibacter actinomycetemcomitans. The Oracare rinse removed a statistically higher percentage of VSCs than chlorhexidine relative to the water control.
Conclusions: These results demonstrate the potent antimicrobial potential of activated chlorine dioxide mouth rinse. Additionally, the chlorine dioxide rinse, when used as an adjunct to conventional periodontal treatment, may help promote periodontal health by neutralizing offending bacterial organisms and byproducts.
Key words: Periodontitis, oral malodor, mouth rinse, chlorine dioxide, volatile sulfur compounds, sodium chlorite, stabilized chlorine dioxide.
Introduction Despite the recognition of microbial species that play prominent roles in the development of dental caries and periodontal disease, most preventive and treatment therapies rely on nonspecific plaque control. When routine plaque control measures such as brushing and flossing prove inadequate, antiplaque agents such as chlorhexidine may be prescribed. Chlorhexidine is considered the “gold standard” of antiplaque agents due to its antimicrobial activity and substantivity. However, its use is often linked to undesirable effects. (1)
Chlorine dioxide is a gas whose oxidizing power is antimicrobial. It has been used in municipal water decontamination as a substitute for chlorine among other applications. A number of mouth rinses on the market have as their active ingredient “stabilized chlorine dioxide.” The term “stabilized chlorine dioxide” can be confusing because these products typically contain little to no chlorine dioxide, but instead contain a product precursor to chlorine dioxide — sodium chlorite. Sodium chlorite rinses do not attain levels of chlorine dioxide equivalent to those found in rinses with active chlorine dioxide, and consequently have less microbial killing power and less oxidation power.
ADDITIONAL READING |Interdisciplinary management of a complex maxillary implant restoration
In a second focus of this study, methods to reduce the presence and production of oral VSCs have long been a focus of research. Most often, the context has been to suppress oral malodor. However, there is evidence that VSCs, particularly methyl mercaptan (CH3SH), have detrimental effects on gingival tissue and are, therefore, more than a cosmetic concern. Ng and Tonzetich determined that VSCs can increase the permeability of intact mucosa, thereby allowing penetration of bacterial products that induce an inflammatory response. (2) Methyl mercaptan negatively impacts collagen metabolism by both decreasing synthesis and increasing degradation, which in turn can undermine the extracellular matrix that supports periodontal tissue. (3)
These observations support the hypothesis that gases such as CH3SH may play a role in both surgical wound healing and periodontal disease by adversely affecting cell function. (4) Makino et al. concluded that VSC levels were significantly associated with periodontal disease progression in a nonsmoking, elderly subject population. (5)
ADDITIONAL READING |Clinical dental advantages of the apically positioned flap
The study reported here was undertaken to test the ability of an activated chlorine dioxide mouth rinse (Oracare) to reduce or eliminate VSC bacterial toxins, and the bacterial producers of those toxins as a precursor to in vivo studies to improve wound healing and the treatment of periodontal disease.
Materials and methods
Bacteria and growth conditions: Bacterial species tested were: P. aeruginosa (ATCC 47085), P. mirabilis (ATCC 14153), Streptococcus mutans (UA159), Lactobacillus acidophilus (ATCC 4356), Porphyromonas gingivalis (ATCC 33277), Aggregatibacter actinomycetemcomitans (ATCC 43718), Tannerella forsythia (ATCC 43037), Treponema denticola (ATCC 35405), Treponema socranskii (ATCC 35535), Prevotella intermedia (ATCC 49046), and Candida albicans (ATCC 64124). Different types of agar plates were necessary to promote growth of the different species. For A. actinomycetemcomitans, S. mutans, P. aeruginosa, and P. mirabilis Brain Heart Infusion (BHI) agar plates were used. For L. acidophilus Lactobacilli MRS agar (Difco) was used, for C. albicans YM agar (Difco) was used, and anaerobic blood agar plates and anaerobic incubation (Difco) were used for P. gingivalis, T. forsythia, and P. intermedia. For the two Treponema species ATCC 1494 Modified NOS medium and anaerobic incubation was used.
Determination of minimum inhibitory concentrations: To test bacterial killing or inhibition in broth (minimum inhibitory concentration; MIC), the same types of media described above were used, except without agar. The media were prepared double strength so that when mixed with an equal volume of the Oracare rinse or chlorhexidine solutions, the media components were at 1x concentration. The bacterial concentration was approximately 4 x 105 CFU/ml. The bacteria were diluted to the desired concentration using the double strength media and 50µl added to wells of a 96-well tissue culture plate. An equal volume of Oracare or water control, always totaling 50µl, was added to the wells containing the bacteria. The range of dilutions tested was from 1:2 to 1:256. The bacteria were incubated at 37°C for as long as necessary to see growth in the growth control well. Anaerobic species were incubated in an anaerobic chamber. The parts per million of active ingredient at the highest dilution at which no bacterial growth was detected was designated the MIC. Since growth of T. denticola was below the threshold of visible turbidity, medium from each well was tested for growth on an agar plate to determine the MIC.
Quantifying removal of volatile sulfur compounds (VSCs): In preparation for comparing the ability of the Oracare rinse with chlorhexidine (0.12% solution; Sunstar Americas, Inc., Chicago, Illinois) for inactivating VSCs, it was determined that P. gingivalis produced the highest levels of VSCs among our anaerobe library and was, therefore, chosen as the organism to generate VSCs for the test system. P. gingivalis was propagated in Thioglycollate Medium (Anaerobe Systems, Morgan Hill, California) and incubated in an anaerobic chamber (85% N2, 10 H2, 5% CO2) at 37°C until turbid (48 to 96 hours).
In order to test the mouth rinses under controlled conditions that would simulate actual use, we chose to measure the reduction in VSCs following a 30-second exposure. P. gingivalis was propagated as described above (except in the absence of agar). The culture was mixed by vortexing and 2 ml was used to saturate a 4.25 cm glass fiber filter (G8; Fisher Scientific, Pittsburgh, Pennsylvania) placed at the bottom of a 50 ml Erlenmeyer flask. The mouth of the flask was covered with aluminum foil to retain VSCs. A baseline reading of VSCs was obtained by removing the foil and placing a two-hole rubber stopper into the mouth of the flask. One hole was connected via tubing to a VSC gas molecule reading halimeter (Interscan Corp., Simi Valley, California) (previously equilibrated to zero parts per billion) while the other hole was left open to facilitate continuous airflow. After the halimeter reached its peak reading (within approximately five seconds), the rubber stopper was removed from the flask and the foil replaced. The peak reading was recorded as the baseline reading and the halimeter was left to re-equilibrate to a stable reading and rezeroed. The filter-containing flask was then rinsed for 30 seconds with 20 ml of a test mouth rinse or water. The rinse was poured off and a postrinse reading taken with the halimeter. The peak reading was recorded as the postrinse level of VSCs. Three to four independent trials were completed. The order in which the mouth rinses were tested was varied with each independent trial to eliminate the possibility of systematic testing bias.
The Oracare was also tested for removal of VSCs compared to “stabilized chlorine dioxide”/sodium chlorite-based mouth rinses and other common rinses: Crest Pro-Health Invigorating Clean (Procter & Gamble Co., Cincinnati, Ohio), Listerine Original Formula (McNEIL-PPC, Inc., Ft. Washington, Pennsylvania), ClōSYS Alcohol-Free Oral Health Rinse (Rowpar Pharmaceuticals, Inc., Scottsdale, Arizona), TheraBreath Oral Rinse (Dr. Harold Katz, LLC, Los Angeles, California), SmartMouth Activated Mouthwash (Triumph Pharmaceuticals, Inc., St. Louis, Missouri), Oxyfresh Patented Zinc Mouthrinse (Oxyfresh Worldwide, Inc., Coeur d’Alene, Idaho), Tom’s of Maine Wicked Fresh (Tom’s of Maine, Kennebunk, Maine), and BreathRx Mouthrinse (Philips Oral Health Care, Los Angeles, California).
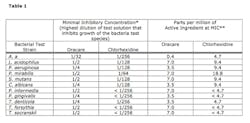
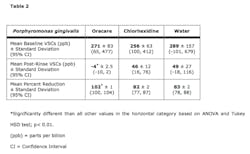
Results The Oracare Rinse was capable of inhibiting growth of all microbial species tested in an MIC assay (Table 1). Since the chlorine dioxide levels of the Oracare Rinse are transitory, gassing out within about an hour, its ability to continuously act on the microbial species for the full time of the MIC testing as chlorhexidine is able to do does not happen with this rinse. Due to this transitory presence of chlorine dioxide levels in the Oracare rinse, it is likely that the Oracare MICs for each microorganism are equivalent to minimum bactericidal concentrations. The direct comparison of Oracare and chlorhexidine MICs revealed a lower MIC for chlorhexidine for each microbial species. However, when examined as parts per million (ppm) of the active ingredients (Table 1) the Oracare was comparable to chlorhexidine for most species, and notably more potent against A. actinomycetemocomitans. These results suggest that by optimizing the peak generation of chlorine dioxide it may be possible for chlorine dioxide based mouthrinses to match or even exceed the antimicrobial activity of chlorhexidine.Next, the Oracare rinse was compared with chlorhexidine for the inactivation of VSCs. The percent reduction in VSCs following a rinse with Oracare was statistically greater than that observed following a rinse with chlorhexidine or with water, the negative control (Table 2). To be sure that reductions in VSCs were not obscured by perfumes or other components within the mouthrinses themselves, each mouth rinse was tested in the absence of VSC-producing bacteria. Halimeter readings for each mouth rinse alone were never greater than three parts per billion.
Discussion
The use of mouth rinses can serve multiple purposes in cosmetic, preventive, and therapeutic realms. The role of chlorhexidine in reducing caries risk is questionable (6,7) though it is useful in plaque control associated with periodontal maintenance. (8) Long-term use is associated with notable adverse effects such as tooth staining and there is increasing awareness of hypersensitivity reactions. (9) Still, chlorhexidine is the standard against which potential alternatives must be compared. In this study, a chlorine dioxide-based mouthrinse, Oracare, was compared to 0.12% chlorhexidine with respect to antimicrobial activity and an ability to inactivate VSCs.
Like chlorhexidine, Oracare had a wide spectrum of activity against Gram-positive and Gram-negative bacterial species, including cariogenic and periodontopathic species. At the concentrations tested, chlorhexidine at 1,200 ppm concentration exhibited lower MICs than Oracare at about 14 ppm concentration for all species tested. As has been mentioned, the generation of chlorine dioxide upon mixing the Oracare components is transient. While preliminary lab data suggested that peak chlorine dioxide concentration is generated sixty seconds after mixing, studies subsequent to our investigation indicated that once diluted the path to peak activity is abrogated. In our experiments dilution was carried out after 30 seconds based on the assumption that generation of chlorine dioxide would continue. When the MIC data are normalized to ppm of active ingredients, Oracare compares favorably with chlorhexidine indicating that optimization of chlorine dioxide generation and exposure may enhance its antimicrobial properties. It is also possible that the differences in in vitro MICs do not directly correspond to in vivo activity. Consequently, future in vivo assessments will be necessary to more precisely define the antimicrobial potential of the chlorine dioxide in comparison to chlorhexidine.
A second property of the Oracare rinse that was analyzed was the ability to inactivate VSCs. The rationale, as described in the Introduction, was based on the hypothesis that VSCs are virulence factors and their removal could accelerate periodontal healing or improve the maintenance of periodontal health. There are multiple means by which the level of oral VSCs can be reduced. The mechanical cleansing effects of using a mouth rinse results in a reduction of the offending bacteria, and temporarily creates conditions less favorable to the production of VSCs by remaining bacteria. It is also possible to further reduce the populations of VSC-producing bacteria through bactericidal or growth inhibitory activities that may be contained within a mouth rinse. Finally, direct inactivation of VSCs is a possibility.
There are several reports in the literature that claim to test the effectiveness of chlorine dioxide mouth rinses at reducing oral malodor as measured by a halimeter or by organoleptic assessment. (10-15) However, these studies, which report variable success, typically employ rinses with sodium chlorite. The active ingredient in Oracare is the gas chlorine dioxide, which is capable of destroying VSCs. The chlorine dioxide in Oracare is generated upon mixing components immediately before use. Thereafter, the chlorine dioxide undergoes decomposition or gassing off in a relatively rapid manner. In our tests, the cleansing activity of the water control was sufficient to remove 83% of VSCs. The chlorhexidine rinse gave nearly identical results as would be expected since it does not chemically inactivate VSCs. VSCs were undetectable after a rinse with Oracare; a reduction that was statistically significant compared to both chlorhexidine and the water control. Additionally, the difference in effectiveness between the chlorine dioxide gas of Oracare and stabilized/precursor forms of chlorine dioxide (sodium chlorite) was evident as none of the mouthrinses using the latter inactivated significantly greater levels of VSCs than the water control. It should be noted as well that because the Oracare rinse removed 100% of VSCs present in the testing system, the ceiling of its capacity to inactivate VSCs was not determined.
The sources of oral volatile sulfur gases most commonly come from dead epithelial cells, leukocytes and salivary substrate. Studies by Kleinberg and Westbay have shown that the mouth must be in an alkaline condition to produce odor. (16) Salivary substrate plays a major role in odor production. Just after a meal is taken, the Gram-positive bacteria are active and make the saliva more acidic. Thus, for a short time the odors go down. The thicker the plaque, the more anaerobic, Gram-negative it is and the more putrefaction and alkalinity that is produced. Although many bacteria produce H2S, the production of CH3SH, especially at high levels, is primarily restricted to periodontal pathogens. Periodontal disease pockets also have an anaerobic, basic pH environment producing putrefaction. (16) Additionally, Yaegaki and Sanada estimated that production of VSCs from tongue coating was four times higher in patients with periodontitis than the control value, and the CH3SH/H2S ratio was also markedly increased. (17) These results strongly suggest that, in addition to periodontal pockets, tongue coating has an important role in VSC production, in particular leading to an elevated concentration of CH3SH, which is more pathogenic than H2S.
Few studies have examined the effects of chlorine dioxide products on oral health measures. A study by Chapek et al. employing RetarDENT and RetarDEX (chlorine dioxide-containing agents that are no longer available) in private practices reported statistically significant reductions in periodontal pocket depths between baseline and recall visits. (18) However, this study did not include a control group. Shinada et al. tested an experimental “chlorine dioxide” mouth rinse, that was in fact sodium chlorite-based, in a double-blind crossover study and reported statistically significant reductions in plaque index, tongue coating index, and recovery of Fusobacterium nucleatum after seven days of using the mouthwash twice daily. (14) There was a trend toward improved gingival index in the “chlorine dioxide” (sodium chlorite) mouth rinse cohort, but the improvement was not statistically significant. These studies reveal a need for further investigation of the potential benefits of chlorine dioxide on periodontal health and a need for greater precision in defining the active ingredients tested to enable accurate comparisons among studies and to a chlorhexidine standard.
Conclusion
The in vitro potential demonstrated for the Oracare rinse supports the hypothesis that it may be particularly efficacious for the reduction of oral malodor and to treat and maintain periodontal health.
Acknowledgments and disclosures
This research was supported by Dentist Select (www.dentistselect.net). Drs. Jeffrey Banas and Min Zhu have extensive experience in oral biology research and no financial interest in Dentist Select. Dr. Richard Downs is a dentist with a private practice in Dubuque, Iowa. He has a financial interest in the company.
Richard Downs, DDS, maintains a private practice of general dentistry in Dubuque, Iowa, and writes and lectures on the link between volatile organic compounds and viral contributions to periodontitis with particular focus on cytokine immune response and its regulation by toxin removal, bacterial destruction, and chlorine dioxide. He is co-founder of the dental products company, Dentist Select, which markets the mouth rinse Oracare and is developing other therapeutic products.
Jeffrey A. Banas, PhD,is a tenured professor at the University of Iowa College of Dentistry, Iowa City, Iowa. The main focus of the lab is investigating the microbiology of dental caries. He received his BS from the University of Notre Dame in 1981 with a major in microbiology, and his PhD from the University of Michigan in 1987 with a major in microbiology. He did his postdoctoral research at the University of Oklahoma Health Sciences Center from 1988 to 1993, where he investigated the role of the Streptococcus mutans glucan-binding protein (GbpA) in cariogenicity.
Min Zhu, DDS, PhD, is a postdoctoral research scholar recently promoted to research specialist at the University of Iowa College of Dentistry, Iowa City, Iowa. He has assisted Dr. Jeff Banas in studies investigating the microbiology of dental caries. Dr. Zhu obtained his DDS from the West China College of Stomatology, Sichuan University, Chengdu, People’s Republic of China, in 1987; his MS from the School of Stomatology, Shanghai Second Medical University, Shanghai, People’s Republic of China, in 1992; and his PhD from the School of Stomatology, Shanghai Second Medical University, Shanghai, People’s Republic of China, in 1995. He completed postdoctoral research from the Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan, in 1998 to 2000, and the Department of Microbiology, Niigata University of Pharmacy, Niigata, Japan, in 2001 to 2002.
Literature cited
1. Varoni E, Tarce M, Lodi G, Carrassi A. Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol 2012;61:399-419.
2. Ng W, Tonzetich J. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J Dent Res 1984;63:994-997.
3. Ratcliff PA, Johnson PW. The relationship between oral malodor, gingivitis, and periodontitis. A review. J Periodontol 1999;70:485-489.
4. Lancero H, Niu J, Johnson PW. Exposure of periodontal ligament cells to methyl mercaptan reduces intracellular pH and inhibits cell migration. J Dent Res 1996;75:1994-2002.
5. Makino Y, Yamaga T, Yoshihara A, Nohno K, Miyazaki H. Association between volatile sulfur compounds and periodontal disease progression in elderly nonsmokers. J Periodontol 2012;83:635-643.
6. James P, Parnell C, Whelton H. The caries-preventive effect of chlorhexidine varnish in children and adolescents: a systematic review. Caries Res 2010;44:333-340.
7. Papas AS, Vollmer WM, Gullion CM, Bader J, Laws R, Fellows J, Hollis JF, Maupomé G, Singh ML, Snyder J, Blanchard P. Efficacy of chlorhexidine varnish for the prevention of adult caries: a randomized trial. J Dent Res 2012;91:150-155.
8. Berchier CE, Slot DE, Van der Weijden GA. The efficacy of 0.12% chlorhexidine mouth rinse compared with 0.2% on plaque accumulation and periodontal parameters: a systematic review. J Clin Periodontol 2010;37:829-839.
9. Pemberton MN, Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. British Dent J 2012;213:547-550.
10. Frascella J, Gilbert RD, Fernandez P, Hendler J. Efficacy of a chlorine dioxide-containing mouth rinse in oral malodor. Compendium 2000;21:241-256.
11. Silwood CJL, Gootveld MC, Lynch E. A multifactorial investigation of the ability of oral health products (OHCPs) to alleviate oral malodor. J Clin Periodontol 2001;28:634-641.
12. Borden LC, Chaves ES, Bowman JP, Fath BM, Hollar GL. The effect of four mouth rinses on oral malodor. Compendium 2002;23:531-548.
13. Peruzzo DC, Jandiroba PFCB, da Rocha Nogueira Filho G. Use of 0.1% chlorine dioxide to inhibit the formation of morning volatile sulphur compounds (VSC). Braz Oral Res 2007;21:70-74.
14. Shinada K, Ueno M, Konishi C, Takehara S, Yokoyama S, Kawaguchi Y. A randomized double blind crossover placebo-controlled clinical trial to assess the effects of a mouthwash containing chlorine dioxide on oral malodor. Trials 2008;9:71.
15. Shinada K, Ueno M, Konishi C, Takehara S, Yokoyam S, Zaitsu T, Ohnuki M, Wright FAC, Kawaguchi Y. Effects of a mouthwash with chlorine dioxide on oral malodor and salivary bacteria: a randomized placebo-controlled 7-day trial. Trials 2010;11:14.
16. Kleinberg I, Westbay G. Salivary and metabolic factors involved in oral malodor formation. J Periodontol 1992;63:768-775.
17. Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients. J Periodontol Res 1992;27:233-238.
18. Chapek CW, Reed OK, Ratcliff PA. Management of periodontitis with oral-care products. Compend Contin Educ Dent 1994;15:740-746.