Predictable tissue repair around diseased dental implants: The missing step
Although the use of dental implants is a reliable treatment option for replacing missing or hopeless teeth, long-term complications that were not known when implants were first utilized have been reported in the literature.1 Implant complications have been categorized into two types: mechanical and/or biologic complications that can present in early or late stages of a dental implant’s life span. Biologic problems include inflammation and/or loss of supporting tissue around a dental implant. Many studies have analyzed different treatment methods for tissue repair around diseased implants with varying degrees of success.2 Most studies have suggested methods for decontaminating the dental implant fixtures prior to tissue grafting.3 Few studies, however, have acknowledged the importance of decontaminating the tissue flap in contact with the diseased implant prior to tissue grafting. This article will briefly address the rationale behind decontamination of the inner lining of the peri-implant soft-tissue flap prior to grafting and suggest a treatment method that can be used to accomplish this goal.
Peri-implantitis has been commonly described in the literature as a pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri‐implant mucosa and progressive loss of supporting bone.4 Plaque/biofilm continues to be the primary etiology of peri-implantitis.5 However, newer studies suggest that titanium or metal particles that come from the implant or prosthesis and embed themselves into the peri-implant tissue may play a role in the pathogenesis of peri-implant diseases.6 This concept, known as implant tribocorrosion, suggests that these particles can be an inflammatory initiator in addition to the commonly accepted bacterial pathogenesis model.7 Tribocorrosion may help explain why many of the treatment efforts around diseased implants that focus solely on detoxification of the implant fixture alone may have failed.
Although the exact mechanism of titanium and metal particles becoming embedded in peri-implant tissue remains unknown, there are a few theories presented in the literature. First, titanium particles might be produced during the insertion of the dental implant into the bone.8 Second, there may be dissolution of titanium from the dental implant into the submucosal plaque as a result of the peri-implantitis disease itself.9 Third, titanium particles might come from the micromotion present at the abutment-implant interface.10 Fourth, metal particles might be produced when performing certain professionally administered hygiene procedures around the dental implant.11
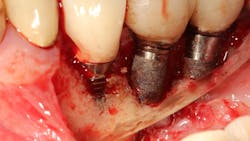
In a recent article discussing two case reports, Nd:YAG and 9.3 micron CO2 lasers were used to detoxify the inner lining of soft tissue surrounding ailing dental implants prior to tissue grafting (figures 1 and 2).12 Lasers can ablate the soft tissue of this particulate (metal) material while killing some of the residual translocated/invasive bacteria, thereby facilitating soft- and hard-tissue healing.
This video demonstrates laser detoxification of the inner lining of the soft-tissue flap, approximating failed dental implant and showing titanium particles embedded into the soft tissue with sparks flying during ablation:
Both the Nd:YAG and 9.3 micron CO2 lasers can have an impact on enhancing soft-tissue healing. Their ability to ablate residual titanium and/or cement from the inner soft-tissue flap lining may help enable the ailing implant sites to successfully heal.
In the two case reports, a laser detoxification approach that included both the implant fixture and the inner lining of the soft-tissue flap were combined with a previously published regenerative surgical algorithm.13 To achieve predictable tissue repair around diseased implants, proper decontamination of both the dental implant and the inner soft-tissue flap is necessary.
All photos courtesy of the author
Editor’s note: This article originally appeared in Perio-Implant Advisory, a newsletter for dentists and hygienists that focuses on periodontal- and implant-References
- Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. White paper for the American Academy of Periodontology. J Periodontol. 2013;84(4):436-443. doi:10.1902/jop.2013.134001
- Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863. doi:10.11607/prd.2571
- Rosen PS, Qari M, Froum SJ, Dibart S, Chou, LL. A pilot study on the efficacy of a treatment algorithm to detoxify dental implant surfaces affected by peri-implantitis. Int J Periodontics Restorative Dent. 2018;38(2):261-267. doi:10.11607/prd.3203
- Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(Suppl 1):S267-S290. doi:10.1002/JPER.16-0350
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S313-S318. doi:10.1002/JPER.17-0739
- Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15. doi:10.1902/jop.2014.140363
- Mombelli A, Hashim D, Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin Oral Implants Res. 2018;29(Suppl 18):37-53. doi:10.1111/clr.13305
- Suárez-Lópe del Amo F, Rudek I, Wagner VP, et al. Titanium activates the DNA damage response pathway in oral epithelial cells: a pilot study. Int J Oral Maxillofac Implants. 2017;32(6):1413-1420. doi:10.11607/jomi.6077
- Safioti LM, Kotsakis GA, Pozhitkov AE, Chung WO, Daubert DM. Increased levels of dissolved titanium are associated with peri-implantitis – a cross-sectional study. J Periodontol. 2017;88(5):436-442. doi:10.1902/jop.2016.160524
- Fretwurst T, Buzanich G, Nahles S, Woelber JP, Riesemeier H, Nelson K. Metal elements in tissue with dental peri-implantitis: a pilot study. Clin Oral Implants Res. 2016;27(9):1178-1186. doi:10.1111/clr.12718
- Harrel SK, Wilson TG Jr, Pandya M, Diekwisch TGH. Titanium particles generated during ultrasonic scaling of implants. J Periodontol. 2019;90(3):241-246. doi:10.1002/JPER.18-0230
- Rosen PS, Froum SH, Froum SJ. A rationale for postsurgical laser use to effectively treat dental implants affected by peri-implantitis: two case reports. Int J Periodontics Restorative Dent. 2020;40(4):561-568. doi:10.11607/prd.4428
- Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.