Background
The COVID-19 pandemic has thrust disinfectant hand sanitizers into the forefront of hygiene rituals to protect against disease transmission. In the United States alone, sales of hand sanitizer jumped 600% in 2020 to become a $1.3 billion industry.1 Most of this craze buying was the result of an article published in March 2020 at the beginning of the pandemic, showing that SARS-CoV-2 had the ability to live on some surfaces for hours and other surfaces for days.2 Unfortunately, much of the data gained from this study has since been deemed both unrealistic and unreproducible. The lab study used large, unrealistic amounts of virus in nonreal-life settings in a vacuum with no air exchange. Since the study’s publication, newer studies have cautioned against concluding that COVID-19 can be transmitted by surfaces infected with the virus.3
Most recent studies have shown that although the virus can be found on surfaces, they are not infectious and do not represent the major source of disease transmission.4 Although the theory of surface transmission had not been fully reproduced by other research teams outside the laboratory setting, the media began to publicize these results, and the surface disinfectant market in the US grew by 30% to become a $4.5 billion industry.
The problem
Because of supply and demand, companies that typically did not make products such as hand sanitizer started to produce it at mass quantity levels with temporary authorization from the Food and Drug Administration (FDA).5 With so many new companies manufacturing hand sanitizer, combined with an unregulated black market, incidents of contamination and toxicity poisoning started to climb. Toxicity issues have been reported as companies began to substitute methanol for isopropyl alcohol or ethyl alcohol, resulting in death.6
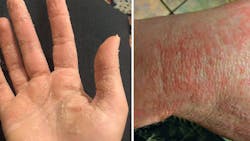
Toxicity has also been reported from hand sanitizer due to overuse. Because most hand sanitizers contain alcohol at a concentration of 60%–95%, common side effects of overuse—especially in children—include dermatitis, dry skin, intestinal issues, and alcohol poisoning (figure 1).8 In the first five months of 2020, more than 9,500 cases of alcohol poisoning were reported in children under age 12 due to alcohol in hand sanitizer.9
A safer alternative
Washing hands with soap and clean water for at least 20 seconds has always been the gold standard of hygiene and safety, particularly for children younger than 12. Even a small amount of alcohol can cause alcohol poisoning in children that is responsible for confusion, vomiting, drowsiness, and, in severe cases, respiratory arrest and death.9
If hand sanitizer must be used, make sure you evaluate the active and inactive ingredients. Although there are no studies on frequency of use for hand sanitizer, a general rule of thumb is once an hour unless your job dictates increased frequency. For the makeup of hand sanitizer, isopropyl alcohol should be in the range of 65%–95%, and ethyl alcohol should ideally be 80% or higher. If you are using hand sanitizer multiple times a day—as is the case with many health-care providers/dental professionals—it would be beneficial for your hand sanitizer to contain skin care elements.
Stella Life makes a hand sanitizer that contains 80% ethanol and hydrogen peroxide with aloe vera, frankincense oil, lavender oil, lemongrass oil, tea tree oil, and vitamin E oil (figure 2). In addition, Stella Life hand sanitizer contains no methanol, dyes, triclosan, parabens, or phthalates, which can lead to dermatitis and toxicity. In conclusion, nothing beats soap and water!
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Terlep S. Hand sanitizer sales jumped 600% in 2020. Purell maker bets against a post-pandemic collapse. Wall Street Journal. January 22, 2021. https://www.wsj.com/articles/hand-sanitizer-sales-jumped-600-in-2020-purell-maker-bets-against-a-post-pandemic-collapse-11611311430
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567. doi:10.1056/NEJMc2004973
- Lewis D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nature. 2021;590:26-28. doi:https://doi.org/10.1038/d41586-021-00251-4
- Ben-Shmuel A, Brosh-Nissimov T, Glinert I, et al. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin Microbiol Infect. 2020;26(12):1658-1662. doi:10.1016/j.cmi.2020.09.004
- Center for Drug Evaluation and Research. Temporary policy for manufacture of alcohol for incorporation into alcohol-based hand sanitizer products during the public health emergency (COVID-19) guidance for industry. US Food and Drug Administration. March 2020. Updated February 10, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/temporary-policy-manufacture-alcohol-incorporation-alcohol-based-hand-sanitizer-products-during
- Kuehn BM. Hand sanitizer poisoning and deaths reported in 2 states. JAMA. 2020;324(12):1130. doi:10.1001/jama.2020.16614
- Durisan hand sanitizer recall due to microbial contamination. US Food and Drug Administration. March 24, 2021. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/durisan-hand-sanitizer-recall-due-microbial-contamination
- Bonner L. CDC report calls attention to hand sanitizer risk in children. Pharmacy Today. 2017;23(5):34. https://www.pharmacytoday.org/article/S1042-0991(17)30602-3/fulltext
- Mahmood A, Eqan M, Pervez S, et al. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci Total Environ. 2020;742:140561. doi:10.1016/j.scitotenv.2020.140561