Metallosis and dental implant failure: The impact of implant breakdown
What is metallosis?
Metallosis is the medical condition involving deposition and buildup of metal debris in the soft tissues of the body. It has been known to occur when metallic components in medical implants, specifically joint replacements, abrade against one another. Metallosis is a type of metal poisoning that can occur as a side effect of joint replacement devices with metal components, such as metal-on-metal hip replacements or other metal implants.1 These devices are made from a blend of several metals, including chromium, cobalt, nickel, titanium, and molybdenum. When the metal parts rub against each other, they release microscopic metal particles into the blood and surrounding tissues. Metallosis develops as these metal ions build up in the bone, muscle, and other tissues around the implant. This can result in loss of bone or other tissue.2 Exposure to metal in the blood and surrounding tissues can lead to several other complications including nerve damage, chronic fatigue, infection, swelling, rashes, tingling sensations, and pain as these metal particles can travel to the brain, heart, eyes, and other organs.3
Dental implant metallosis and failure
Teeth and dental implants have different types of attachment to the body, giving them different breakdown characteristics when they encounter bacteria. Teeth have a periodontal ligament, many supracrestal fibers, and true epithelial/connective tissue attachment. Dental implants, on the other hand, have only a couple of supracrestal fibers, a pseudo tissue attachment, and a thin titanium oxide layer (TiO2) that attaches the implant to bone but is susceptible to breakdown when confronted by bacteria. This layer of TiO2 is vital to the process of osseointegration and forms a protective barrier to prevent exposure of the implant to the surrounding tissues.4 In vitro experiments have shown that the action of bacteria on the implant surface releases titanium particles and ions by disrupting the TiO2 layer.
Metallosis, caused by this titanium breakdown, can contribute to dental implant failure when released titanium particles/ions into surrounding tissues cause inflammatory foreign body reactions. This process is triggered by oral bacteria and can be exacerbated by mechanical forces.5 These breakdown products behave as haptens, generating a hypersensitive reaction that involves the release of inflammatory mediators that can cause urticaria, eczema, oedema, redness, and pruritus of the skin or mucosa, either localized or at distant sites.6 The literature on dental implant breakdown resulting in localized or generalized reactions is confined to case reports, and long-term, well-controlled research trials are needed to show definitive causation.
How do dental implants break down causing metallosis?
No. 1: Corrosion.
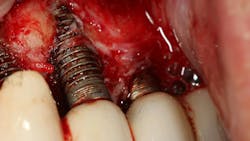
Corrosion is defined as the deterioration of a metal due to interaction (electrochemical attack) with its environment, which results in the release of ions into the surrounding microenvironment.7 Bacteria such as Streptococcus mutans and Porphyromonas gingivalis have been shown to remove the TiO2 layer around dental implants (figure 1). This exposes the dental implant body to the oral environment, leading to breakdown and metal particle release (corrosion).8 Bacterial attachment can occur within 30 minutes of implant placement. Corrosion can occur within two days after bacterial attachment, although this process typically can take longer.9 Once corrosion occurs, more bacteria can attach to the implant, which results in further corrosion. These ions/particles produced by corrosion can cause an inflammatory response in the soft tissues around dental implants, which can result in dental implant failure.10 Corrosion has been shown to affect all dental implant surfaces, including commercially pure titanium, titanium alloys, as well as zirconium.11
No. 2: Micromovement (fretting)
Micromovement of the dental implant, also known as corrosion fretting, can result in loss of the TiO2 layer around the dental implant, leading to ion/particle release.12 This, again, can result in a similar inflammatory process as described above and implant loss.
No. 3: Tribocorrosion
Tribocorrosion is the result of dental exposure to both acid from the mouth as well as mechanical forces that cause breakdown of the implant body and titanium particle release. The acid can result from bacterial biofilm, exposure to acidic foods/liquids, and/or oral hygiene products.13 Because there is a synergistic effect of occlusal loading and acid exposure, tribocorrosion negatively affects the surface of titanium dental implants to a greater degree than that of corrosion or fretting individually.
No. 4: Physical trauma
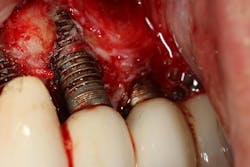
Degradation of the implant body can occur at any time, from when the implant is physically inserted into the bone to the hygiene phase when the implants are mechanically debrided with hand scalers or ultrasonic/piezoelectric devices.14 In addition, some authors advocate the technique known as “implantoplasty,” where the surface of dental implants affected by peri-implant disease are plastied with high-speed handpiece burs to remove bacterial biofilm (figure 2).15 This can result in the release of titanium particles with similar inflammatory breakdown effects.16
No. 5: Manufacturing inaccuracy
A recent case series found that dental implants examined from various manufacturers were contaminated with titanium debris (particles) within the internal aspect of the implant fixture when visually inspected at 30x magnification. Titanium is considered difficult to machine and poorly suited to many applications that involve friction, lubrication, and wear such as dental implants and their inside screw threads.17 Titanium chip formation (particles) is known to occur, and these particles may remain unless effectively removed by the manufacturer.18 If not removed, these particles can travel and cause inflammatory reactions, leading to tissue breakdown and possible failure.
Conclusion
Although further well-controlled studies are needed to pinpoint a causal relationship between titanium implant breakdown/particle release and dental implant failure, many studies have shown a pathologic mechanism to bone loss and subsequent dental implant failure.19 Given the information available, it seems logical that the body’s response to titanium particles and ions around dental implants would be similar to that around failing orthopedic prosthetic joints.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27(12):1533-1544. doi:10.1002/jbm.820271210
- Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88(6):1183-1191. doi:10.2106/JBJS.D.02916
- Harris A, Johnson J, Mansuripur PK, Limbird R. Cobalt toxicity after revision to a metal-on-polyethylene total hip arthroplasty for fracture of ceramic acetabular component. Arthroplast Today. 2015;1(4):89-91. doi:10.1016/j.artd.2015.09.002
- Sul YT. The significance of the surface properties of oxidized titanium to the bone response: special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials. 2003;24(22):3893- doi:10.1016/s0142-9612(03)00261-8
- Wilson TG Jr. Bone loss around implants–is it metallosis? J Periodontol. 2021;92(2):181-185. doi:10.1002/JPER.20-0208
- Olmedo D, Tasat D, Duffó G, Cabrini R, Guglielmotti M. Systemic and Local Tissue Response to Titanium Corrosion. IntechOpen; 2012. doi:10.5772/32500
- Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(2):268-282. doi:10.2106/00004623-199802000-00015
- Souza JCM, Henriques M, Oliveira R, Teughels W, Celis JP, Rocha LA. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling. 2010;26(4):471- doi:10.1080/08927011003767985
- Sridhar S, Wilson TG, Jr, Palmer KL, et al. In vitro investigation of the effect of oral bacteria in the surface oxidation of dental implants. Clin Implant Dent Relat Res. 2015;17(Suppl 2):e562-e doi:10.1111/cid.12285
- Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15. doi:10.1902/jop.2014.140363
- Siddiqui DA, Guida L, Sridhar S, Valderrama P, Wilson TG Jr, Rodrigues DC. Evaluation of oral microbial corrosion on the surface degradation of dental implant materials. J Periodontol. 2019;90(1):72-81. doi:10.1002/JPER.18-0110
- Geringer JD, Demanget N, Pellier J. From acid etching treatments to tribocorrosive properties of dental implants: do some experimental results on surface treatments have an influence on the tribocorrosion behaviour of dental implants. J Phys D: Appl Phys.2013;46(4):404005. doi:10.1088/0022-3727/46/40/404005
- Gaur S, Agnihotri R, Albin S. Bio-tribocorrosion of titanium dental implants and its toxicological implications: a scoping review. ScientificWorldJournal. 2022;2022:4498613. doi:10.1155/2022/4498613
- Yen Nee W, Raja Awang RA, Hassan A. Effects on the titanium implant surface by different hygiene instrumentations: a narrative review. Cureus. 2022;14(10):e30884. doi:10.7759/cureus.30884
- Monje A, Pons R, Amerio E, Wang HL, Nart J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: a retrospective study. J Periodontol. 2022;93(1):110-122. doi:10.1002/JPER.21-0103
- Toledano-Serrabona J, Bosch BM, Díez-Tercero L, et al.Evaluation of the inflammatory and osteogenic response induced by titanium particles released during implantoplasty of dental implants. Sci Rep.2022;12(1):15790. doi:10.1038/s41598-022-20100-2
- Wiklund U, Hutchings IM. Investigation of surface treatments for galling protection of titanium alloys. Wear. 2001;250(1-12):1034-1041. doi:10.1016/S0043-1648(01)00730-X
- Xi Y, Bermingham M, Wang G, Dargusch M. Finite element modeling of cutting force and chip formation during thermally assisted machining of Ti6Al4V alloy. J Manuf Sci Eng. 2013;135(6):188-197. doi:10.1115/1.4025740
- Fretwurst T, Nelson K, Tarnow DP, Wang HL, Giannobile WV. Is metal particle release associated with peri-implant bone destruction? An emerging concept. J Dent Res. 2018;97(3):259-265. doi:10.1177/0022034517740560
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.
Chandur P.K. Wadhwani, BDS, MSD
Chandur P.K. Wadhwani, BDS, MSD, is in private practice limited to prosthodontics in Bellevue, Washington. He is an affiliate assistant professor in the department of restorative dentistry at the University of Washington School of Dentistry in Seattle, Washington; associate professor at the University of Oregon Health Science University in Portland, Oregon; and assistant professor of the advanced education program in prosthodontics at Loma Linda University in Loma Linda, California.
Updated April 21, 2023