Laboratory-produced human bone to be transplanted in humans: Bonus BioGroup launches first clinical trial to transplant live bone grafts into deficient human facial bones
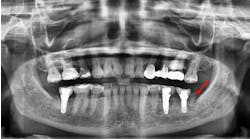
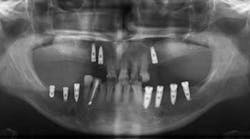
Bonus BioGroup announces it has commenced a clinical trial for the repair of human facial bone deficiency, including upper or lower jawbone cavitation by transplantation of live human bone grafts produced in the company's manufacturing facility at Matam High-Tech & Business Park, Haifa, Israel.
ADDITIONAL READING |Periodontal regeneration: a look at the current and future landscape
Bonus BioGroup produces live human bone grafts comprising of cells harvested from patients' own fat tissue, in a personalized medicine approach. Its novel proprietary technology for the manufacturing of these cells combines multiple disciplines, such as material and tissue engineering, biology and regenerative medicine.
ADDITIONAL READING |Guided tissue regeneration: background to current indications and applications
Enrollment of the first patient and execution of the first stages of this bone void-filling clinical trial have been successfully performed; a fat tissue sample was obtained by clinical biopsy and transported within several hours to Bonus BioGroup's manufacturing facility. There, cells were extracted from the sample tissue and the process of generating live human bone graft commenced.
The manufacturing of the live bone graft is expected to be completed within approximately one month, following which a ready-to-use injectable bone graft will be in place and maintained in stand-by at the manufacturing facility. On the scheduled day of transplantation, it shall be transported to the medical site to be transplanted back into the patient's jawbone.
As the manufactured bone grafts originate from the same patients receiving the grafts, Bonus BioGroup's live human bone grafts are anticipated to induce full immunological tolerance. The biological similarity between the patients' body and their autologous grafts is expected to be identified by the immune system and thereby prevent graft rejection — a common phenomenon in allograft (foreign donor tissue) transplantation.
In the upcoming weeks, Bonus BioGroup hopes to enroll all additional participants in this clinical trial. Fat tissue samples shall be collected from these patients and utilized to repair their various facial bone deficits. This clinical trial is intended to conclude within one year of the graft transplantations, with a primary endpoint to evaluate the safety and efficacy of Bonus BioGroup's live human bone grafts. Initial results for each patient shall become available four months after their transplantation.
Bonus BioGroup's manufacturing facility was designed and established in compliance with the European Good Manufacturing Practice (GMP) and meets all advanced therapy regulations in regard to the production of clinical-grade cellular products suitable for human therapy. Its GMP-grade operational manufacturing facility allows Bonus BioGroup full control over the entire process of bone graft production, without being reliant upon collaborations with other parties.