Abstract
Type 2 diabetes and periodontal disease are separate inflammatory diseases that augment each other. A complication such as type 2 diabetes makes people more likely to have problems with oral health and has been shown to promote periodontal disease. In turn, periodontal disease appears to exacerbate type 2 diabetes. Cellular inflammation from immune cells appears to be the common biological denominator that is the link between type 2 diabetes and periodontal complications.
Introduction
Inflammatory diseases share a common biological denominator which is the ability to produce pro-inflammatory cytokines that play an important role in physiologic regulation of many biological activities. However, inflammatory diseases sometimes produce cytokines at levels that have pathological consequences.
The role of cellular inflammation
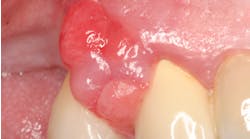
Diabetes affects an increase in periodontal disease through impaired defense mechanisms involving micro- and macrovasculature changes that lead to anincreased susceptibility to infection and reduced healing capacity.1 Insulin resistance is the primary driving force in type 2 diabetes. Adipocytes and macrophages by synthesizing excessive cytokines directly and indirectly affect glucose metabolism2 and hyperglycemia. This results in an increase in the levels of advanced glycation end products (AGE), which make endothelial cells and monocytes more susceptible to inflammation and lead to an increase in vascular permeability.3 Inflammation is further exacerbated when a shift toward an increased incidence of periodontal pathogens in diabetic patients4 causes an alteration from a symbiotic to a dysbiotic microflora, resulting in a further increase in vascular permeability (figure 1).5
An increased vascular permeability results in a dissemination of oral bacteria into the bloodstream6 and up to a fourfold increase in systemic entry of periodontal biofilm products (endotoxins) into the host circulatory system during mastication.7 These periodontal biofilm products stimulate a variety of host responses, including an increase in interleukin-1 beta, interleukin-6, interleukin-8, prostaglandins, and tumor necrosis factor-alpha,8 which have an effect on diabetes.
Interleukin-1 and interleukin-6 have been shown to impair the release of insulin from pancreatic beta cells.9,10 Interleukin-8 produces insulin resistance via the inhibition of insulin-induced Akt phosphorylation in adipocytes.11 Prostaglandins functioning independent of or in combination with IL-6 may affect insulin sensitivity and related adipocyte inflammation.12 Systemic TNF-α impairs glucose uptake and metabolism by altering insulin signal transduction, resulting in a low-grade systemic inflammation and insulin resistance in skeletal muscle.13 These interactions induce or perpetuate activation of the intracellular pathways, such as the I-kappa-B, I-kappa-B kinase-β, nuclear factor-kappa B, and the protein c-Jun N-terminal kinase axes, all of which are associated with insulin resistance.14
Research
Periodontal disease and diabetes are interrelated, but research shows variable results for diabetic control when evaluating periodontal disease treatment. One study showed scaling and root planing did not decrease fasting blood glucose levels.15 A study of subjects with type 2 diabetes who underwent scaling and root planing and received adjunctive doxycycline therapy demonstrated significant improvement in periodontal health but only a nonsignificant reduction in HbA1c values.16 Other studies confirmed a positive metabolic response to nonsurgical periodontal treatment.17 Further studies are needed to find ways to control the periopathogens, decrease localized and systemic inflammation, reduce periodontal pockets, and enhance wound healing to decrease diabetic markers.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
1. Daniel R, Gokulanathan S, Shanmugasundaram N, Lakshmigandhan M, Kavin T. Diabetes and periodontal disease. J Pharm Bioallied Sci. 2012;4(Suppl 2):S280-S282. doi:10.4103/0975-7406.100251
2. Sibner JA. The inflammatory origins of periodontal disease and diabetes: a framework for understanding clinical outcomes. Ineedce.com website. ineedce.com/courses/2363/PDF/1210cei_sibner_web.pdf Published October 2012. Accessed June 29, 2016.
3. Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94(1):10-21.
4. Ohlrich EJ, Cullinan MP, Leichter JW. Diabetes, periodontitis, and the subgingival microbiota. J Oral Microbiol. 2010;2. doi:10.3402/jom.v2i0.5818
5. Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55(1):36-47.
6. Abranches J, Zeng L, Bélanger M, et al. Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol. 2009;24(2):141-145. doi:10.1111/j.1399-302X.2008.00487.x
7. Geerts SO, Nys M, De MP, et al. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol. 2002;73(1):73-78.
8. Scannapieco FA. Periodontal inflammation: from gingivitis to systemic disease? Compend Contin Educ Dent. 2004;25(7 Suppl 1):16-25.
9. Nackiewicz D, Dan M, He W, et al. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia. 2014;57(8):1645-1654. doi:10.1007/s00125-014-3249-1
10. Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997;82(12):4167-4170.
11. Kobashi C, Asamizu S, Ishiki M, et al. Inhibitory effect of IL-8 on insulin action in human adipocytes via MAP kinase pathway. J Inflamm (Lond). 2009;6:25. doi:10.1186/1476-9255-6-25/p>
12. Yasui M, Tamura Y, Minami M, et al. The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PloS One. 2015;10(8):e0136304. doi:10.1371/journal.pone.0136304
13. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54(10):2939-2945.
14. Santos Tunes R, Foss-Freitas MC, Nogueira-Filho GR. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35.
15. Alshehri FA, Javed F. Impact of scaling and root planing on clinical periodontal status and glycemic levels in prediabetic patients. Interv Med Appl Sci. 2015;7(1):17-21. doi:10.1556/IMAS.6.2014.004
16. Promsudthi A, Pimapansri S, Deerochanawong C, Kanchanavasita W. The effect of periodontal therapy on uncontrolled type 2 diabetes mellitus in older subjects. Oral Dis. 2005;11(5):293-298.
17. Hungund S, Panserlya BJ Reduction in HbA1c levels following non-surgical periodontal therapy in type 2 diabetic patients with chronic generalized periodontitis: a periodontist’s role. J Indian Soc Periodontol. 2012;16(1):16-21. doi:10.4103/0972-124X.94598

![Figure 1: The loss of epithelial cells (arrow) from host inflammation exposes the circulatory system [capillary to the right of the biofilm] to an increased entry of bacteria, biofilm products, and host inflammatory cytokines into the host blood stream. Figure 1: The loss of epithelial cells (arrow) from host inflammation exposes the circulatory system [capillary to the right of the biofilm] to an increased entry of bacteria, biofilm products, and host inflammatory cytokines into the host blood stream.](https://img.perioimplantadvisory.com/files/base/ebm/pia/image/2022/03/Figure1.623e2e28aaa87.png?auto=format,compress&fit=max&q=45&w=250&width=250)