Treatment of exposed dental implant surfaces with the minimally invasive bone grafting technique: SMART
As dental implant placement has become ubiquitous in dental practice, interest has increased in treatment options to manage peri-implantitis and other complications. In cases of exposed implant surfaces, a subperiosteal minimally invasive bone grafting technique (SMART) has delivered consistent outcomes without flaps, membranes, or tenting screws, and fewer complications—a promising alternative for the management of peri-implant bone loss.
Since dental implant placement has become a ubiquitous procedure in dental practice, there has been increased interest in treatment options to manage peri-implant diseases and their complications.
Although opinions vary among authors, proposed definitions of peri-implantitis have included the presence of inflammation, increased probing depths, bleeding upon probing, suppuration, and bone loss. (1, 2) A recent peri-implantitis classification has proposed the inclusion of implants exhibiting bone loss that could be deemed of iatrogenic origin. (3) Among the factors that may contribute to these unexpected outcomes are the presence of a thin labial plate, remodeling of a grafted site, or improperly placed implants. The risks associated with flapless, immediate, postextraction implant placement must also be considered in this regard. Despite its popularity, this is a technique-sensitive procedure that may result in buccal implant placement. (4, 5)
A consensus with regard to the preferred method of therapy for peri-implant diseases does not exist. Various protocols for the management of peri-implantitis have been proposed, many of them combining surface decontamination and traditional guided bone regeneration (GBR) principles. (6) Reosseointegration of exposed implant surfaces appears possible, however, as recently reported in a human model. (7) Defect configuration seems to play a role in the response to peri-implantitis therapy, which may be improved in situations where bone loss is restricted to the buccal surface of the implant. (8)
A recent publication in the International Journal of Periodontics & Restorative Dentistry presented the results of a prospective case series utilizing a subperiosteal minimally invasive esthetic ridge augmentation technique (SMART). The SMART technique was used to treat 60 sites on 21 patients, with a follow-up period ranging from four to 30 months, and human histologic results. Five treatment categories were reported, including the treatment of exposed dental implant surfaces. (9)
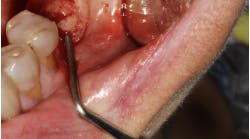
The SMART procedure is initiated with a remote incision from which a confined subperiosteal tunnel is developed, using specially designed instruments. Once the exposed implant surface is accessed, a subperiosteal pouch with a clearly defined perimeter is created to house the bone grafting material. Chlorhexidine gluconate and hydrogen peroxide are subsequently used for implant surface decontamination.
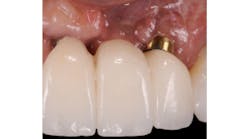
Anorganic bovine bone xenograft particles are mixed with recombinant human platelet-derived growth factor (rhPDGF-BB). The combination is delivered into the subperiosteal pouch with a specially configured carrier and compacted. No marrow penetration, space-making devices, or membranes are required. Particle aggregation within the pouch results in horizontal bone augmentation lateral to the exposed implant surface (figure 1).
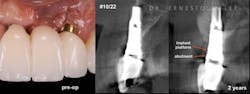
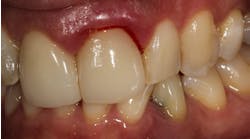
Figure 1: Patient reported discomfort and periodic fistula on labial mucosa of Nos. 10/22. Existing bridge is 12 years old, with implant Nos. 9/21 submerged. Previous history of failed implants/GBR in same area. Buccal plate regeneration was achieved with SMART bone graft. Stable bone volume and no symptoms after two years.
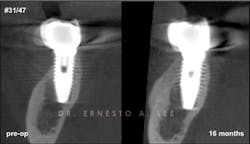
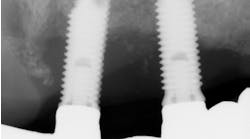
Figure 2: Implant in second molar area was placed 15 years ago. Labial bone remodeled over the years, creating a soft-tissue concavity, and the patient complained of food impaction. Buccal plate regeneration with SMART bone graft exhibits volume stability after 16 months.
The average horizontal augmentation achieved with the SMART method for all treatment categories was 5.11 mm (SD=0.76). The mean horizontal augmentation of edentulous ridges using the SMART method was 6.47 mm (SD=1.40). This compares favorably with outcomes reported by Urban and colleagues in 2013, Proussaefs and Lozada in 2006, and Pieri et al. in 2008. (10–12) Additionally, human histology demonstrated xenograft particles surrounded by newly formed bone. Histomorphometric analysis revealed a 52% bone content in the sample.
Bone augmentation over the exposed implant surfaces has been consistently achieved with the SMART technique. The augmented volumes have remained dimensionally stable, and no symptoms have been reported (figure 2).
The SMART approach has delivered consistent outcomes with no flaps, no membranes, no tenting screws, and fewer complications. It, therefore, constitutes a promising alternative for the management of peri-implant bone loss. This is, however, a technique-sensitive procedure for which training and specially designed instrumentation are required.
Author's note: The SMART method—and devices associated with the method—are protected by one or more patent-pending applications. If you wish to learn more about SMART, or to register for our October 13 and 14 training and certification course, please visit drernestolee.com/ or contact [email protected].
References
1. Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, eds. Proceedings of the First European Workshop on Periodontology. London: Quintessence; 1994:365-369.
2. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 Suppl):286-291. doi: 10.1111/j.1600-051X.2008.01274.x.
3. Sarmiento HL, Norton MR, Fiorellini JP. A classification system for peri-implant diseases and conditions. Int J Periodontics Restorative Dent. 2016;36(5):699-705. doi: 10.11607/prd.2918.
4. Chen ST, Darby IB, Reynolds EC, Clement JG. Immediate implant placement postextraction without flap elevation. J Periodontol. 2009;80(1):163-172. doi: 10.1902/jop.2009.080243.
5. Morton D, Chen ST, Martin WC, Levine RA, Buser D. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. 2014;29(Suppl):216-220. doi: 10.11607/jomi.2013.g3.
6. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: A consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863. doi: 10.11607/prd.2571.
7. Fletcher P, Deluiz D, Tinoco EM, Ricci JL, Tarnow DP, Tinoco JM. Human histologic evidence of reosseointegration around an implant affected with peri-implantitis following decontamination with sterile saline and antiseptics: A case history report. Int J Periodontics Restorative Dent. 2017;37(4):499-508. doi: 10.11607/prd.3037.
8. Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010;37(5):449-455. doi: 10.1111/j.1600-051X.2010.01540.x.
9. Lee EA. Subperiosteal minimally invasive aesthetic ridge augmentation technique (SMART): A new standard for bone reconstruction of the jaws. Int J Periodontics Restorative Dent. 2017;37(2):165-173. doi: 10.11607/prd.3171.
10. Urban IA, Nagursky H, Lozada JL, Nagy K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: A prospective case series in 25 patients. Int J Periodontics Restorative Dent. 2013;33(3):299-307. doi: 10.11607/prd.1407.
11. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: Clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32(5):237-247.
12. Pieri F, Corinaldesi G, Fini M, Aldini NN, Giardino R, Marchetti C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: A 2-year prospective study. J Periodontol. 2008;79(11):2093-2103. doi: 10.1902/jop.2008.080061.